Ground Zero Pharmaceuticals, Inc. provides integrated consultation in regulatory affairs, toxicology, pharmacology, clinical development, biostatistics, CMC, medical writing, electronic submissions, and project management for pharmaceutical, biologic, medical device and combination product programs. Our services extend beyond our FDA focus to preparing submissions for European regulators, dossiers to Health Canada, and interactions with the Australian TGA.
GZP provides specialized consulting in many therapeutic areas. Some areas of expertise include:
- Antithrombotics
- Antiemetics
- Anti-inflammatory/Analgesics
- Antivirals/Anti-infectives/AIDS
- Antifungals
- Cardiovascular
- Dermatologicals
- Diagnostic Imaging and Susceptibility Testing
- Fertility/IVF
- Genito-Urinary
- Hematologicals
- Immunologicals
- Oncology
- Ophthalmologicals
- Oral Healthcare
- Neuropharmacologicals
- Somatic Cell Therapies
- Vaccines
We have rapid initiation and execution phases, and the team you meet will be the team that actually follows through to help make your program successful. A highly trained GZP project manager serves as the primary contact person for each client, ensuring coordination of information and efforts and providing a central resource for rapid responses.
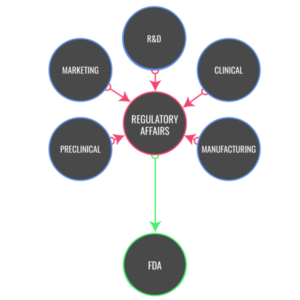
The inefficiency and relative lack of benefit for the client in the usual CRO model for consulting services are demonstrated by inordinate delays in the conduct of clinical trials, massive cost overruns and lack of attention to detail in CRO teams with conflicting priorities. This results in delays in submissions and manufacturing; clinical or technological questions raised late in the review process; and most importantly, delays in product approval. GZP emphasizes a proactive approach to these issues from the very first interactions with our clients and partners. If you demand targeted management, regulatory and product development consulting, prompt attention to detail, individual consideration and first-rate quality, then choose Ground Zero Pharmaceuticals, Inc.

